Website:
Telic
Website:
Telic
Group: Telic Group
Catalog excerpts

TECHNICAL DATA SHEET Ultrasound transmission sterile gel Transonic Product description Sterile ultrasound gel is intended for general use as a transmission media for acoustically coupling a transducer to a human body surface during external therapeutic and diagnostic ultrasound imaging procedures. It is placed on the patient’s skin or on the transducer prior to initiating an ultrasound examination. The sterile product is recommended for diagnostic and therapeutic ultrasound applications when sterility is indicated. Does not irritate skin. Does not contain salt. Does not damage the transducer. It spreads out easily and uniformly. This product is free to natural latex, phthalatos and animal or biological origin compounds. Technical specifications Ingredients • Aqua (water), Propylène Glycol, Carbomer, Sodium Hydroxide, Diazolidinyl Uréa, Methylparaben, Propylparaben, Disodium EDTA. Gel composition: Water-based gel with polymers. Sachet: PET/Pet-metalized/PE film Thermo-sealed pouch: • Cellulose paper, medical grade Transparent PA/PP film This document is the property of Telic, S.A.U. Transmission to third-parties or non-authorized copies are no
Open the catalog to page 1
TECHNICAL DATA SHEET Physico-chemical properties • Colour, Odour, Appearance: Colourless or slightly yellowish, transparent and viscous gel. Odour to its raw materials (it does not contain perfume). Microbiological properties • Sterile according the normative EN 556-1 standard Conclusion: The product is biocompatible. Sterilization and expiry date • Sterile product by radiation. Shelf life: 2 years upon manufacturing date. Keep protected from direct sunlight / the outside. Single use product. No maintenance required. Double packaging, monodose, 20 ml, 48 unit service box. Apply over the...
Open the catalog to page 2
TECHNICAL DATA SHEET Regulatory information TELIC, S.A.U. guarantees that this product is in conformity with Regulation (UE) 217/745 and that it has been manufactured following the directives of the Quality Assurance System certified as ISO 13485. This product is classified as: • Class I sterile product according to Annex XI of Regulation (UE) 2017/745, rule 1. GMDN code: 58735 – Coupling gel, sterile. EMDN code: Z11040185 (Ultrasound scanners-consumables) This document is the property of Telic, S.A.U. Transmission to third-parties or non-authorized copies are not allowed 3-3
Open the catalog to page 3All Telic catalogs and technical brochures
-
CUp Electrodes
3 Pages
-
Surface electrodes
2 Pages
-
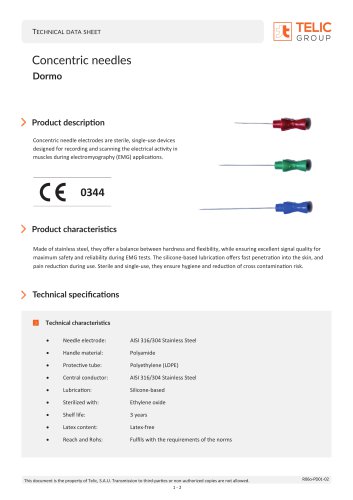
Concentric needles
2 Pages
-
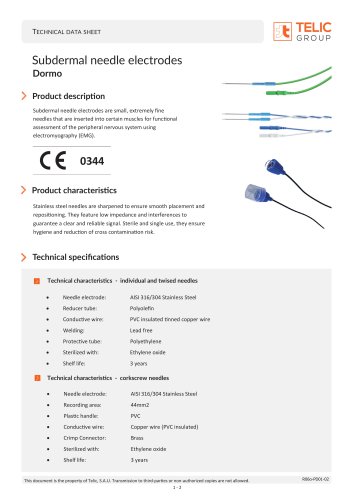
Corkscrew subdermal needles
2 Pages
-
Conductive gel
3 Pages
-
Telic Groupe
27 Pages
-
Blayco dermal curette
2 Pages
-
Blayco biopsy punches
3 Pages
-
Blayco-Pad
2 Pages
-
Skin marker
2 Pages
-
Desfi-Dormo ED-1010
3 Pages
-
Dormo-Strip
3 Pages