Catalog excerpts
INFECTIOUS SEROLOGY – VIROLOGY – HERPES SIMPLEX VIRUS Herpes Simplex Virus ELISA and MICROBLOT-ARRAY kits are optimized and validated for detection of IgG, including their avidity, and IgM antibodies in human serum, plasma or cerebrospinal fluid Diagnostic kits are intended for professional use in the laboratory. Enzyme immunoassays for the diagnostics of Herpes simplex virus infectio
Open the catalog to page 1
INFECTIOUS SEROLOGY – VIROLOGY – HERPES SIMPLEX VIRUS Herpes simplex virus exists in two types (HSV-1 and The primary infection is always accompanied by spe- HSV-2) and belongs to the herpesvirus family. Both va- cific IgM antibodies which are already produced one rieties have common antigens and cross-react during week after infection and persist for approximately serological tests. Herpes is transmitted by means of 6 weeks. The specific IgM antibodies may or may not be droplet infection or during close contact with an infec- found during reactivation. Specific IgA antibodies occur ted...
Open the catalog to page 2
ELISA Summary protocol Test principle The assays are based on a sandwich type of ELISA method. Test steps Pipette Controls and diluted 2. Dilution of samples – serum/plasma 1:101 (10 μl + 1 ml) samples 100 μl – Including blank Aspirate and wash the wells 5 times Antigens EIA HSV 1+2 Mixture of inactivated and purified HSV-1 and HSV-2 strains EIA HSV 1 Purified and inactivated HSV-1 antigen with a high content of specific immunodominant Add Conjugate 100 μl – Including blank Aspirate and wash the wells 5 times Add 100 μl Substrate (TMB-Complete) – Including blank Incubate 30 min. at 37°C Add...
Open the catalog to page 3
Clinical application - Screening test for the detection of specific IgG and IgM antibodies in human serum, plasma or cerebrospinal fluid - Checking of therapy results using the semiquantitative determination - Disease stage diagnosis User comfort - Ready-to-use components - Colour-coded components - Interchangeable components - Breakable colour-coded microplate strips - CUT-OFF included - Semiquantitative evaluation of results (Index of Positivity) Advantages - High diagnostic specificity and sensitivity - High reproducibility - High dynamics of antibody response - Identical assay procedure...
Open the catalog to page 4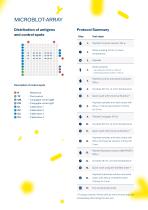
Distribution of antigens and control spots Protocol Summary Step 6 1. Test steps Pipette Universal solution 150 pi Strips soaking 10 min. at room temperature Aspirate Dilute samples 4. - serum/plasma 1:51 (10 pl + 500 pl) Aspirate samples and wash strips with © 8. 150 pl of Universal solution 3-times Description of control spots Pipette Controls and diluted samples 6. Incubate 30 min. at room temperature 7. Quick wash with Universal Solution* 9. Pipette Conjugate 100 pl © 10. Incubate 30 min. at room temperature Quick wash with Universal Solution* Aspirate samples and wash strips with © 12....
Open the catalog to page 5
Description of antigens Antigen Glycoprotein C-1 specific for Herpes simplex 1 virus Glycoprotein C-2 specific for Herpes simplex 2 virus Early antibody production Glycoprotein D-1 specific for Herpes simplex 1 virus Glycoprotein D-2 specific for Herpes simplex 2 virus serves to capture and entry of the virus into a potential host cell; stimulates high production of neutralizing antibodies, high similarity between HSV-1 and -2 Glycoprotein G-1 specific for Herpes simplex 1 virus Glycoprotein G-2 specific for Herpes simplex 2 virus Appropriate for differentiating between HSV-1 and -2...
Open the catalog to page 6
INFECTIOUS SEROLOGY – VIROLOGY – HERPES SIMPLEX VIRUS Ordering information ELISA Cat. No. CKS negative (according to the list on www.testlinecd.cz) CKS positive (according to the list on www.testlinecd.cz) SmartEIA kits are designed for automated processing using the Agility® analyser. MICROBLOT-ARRAY Product TestLine Clinical Diagnostics Ltd. Krizikova 68, 612 00 Brno, Czech Republic +420 549 121 203 management system standards Company is certified to the quality
Open the catalog to page 8All TestLine Clinical Diagnostics catalogs and technical brochures
-
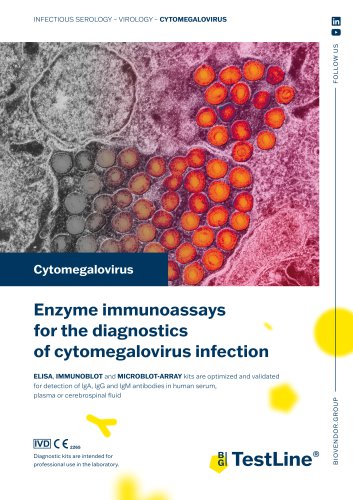
Cytomegalovirus
8 Pages
-
Parvovirus B19
4 Pages
-
Veterinary Portfolio
20 Pages
-
COVID-19
12 Pages
-
Respiratory viruses
8 Pages