
Catalog excerpts

Thermo Scientific Reagents, Solvents and Accessories Part of Thermo Fisher Scientific Gas Chromatography High Performance Liquid Chromatography Mass Spectrometry
Open the catalog to page 1
Thermo Scientific Products for GC and HPLC Derivatization Reagents for Specific Functional Groups 1 Derivatization Reagents for Drug-of-Abuse 2-3 Specialized Products and Reagents 20 HPLC Ion Pair Reagents 24-40 Binding and Elution Buffers for Affinity Purification 25 HPLC Ion Pair Reagents 26 HPLC/Spectrophotometric Grade Solvents 27 Derivatization and Visualization Reagents for HPLC 28-30 Amino Acid Analysis 32-41 Sample Preparation and Hydrolysis 34 Fluorometric Detection Reagents for Amino Acids 36 Ninhydrin Detection Reagents for Amino Acids 37-38 High-Purity Pre-Column Derivatization...
Open the catalog to page 2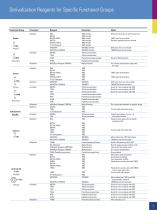
Derivatization Reagents for Specific Functional Groups Functional Group Silylation BSA BSTFA BSTFA+TMCS Amides MSTFA O MSTFA+TMCS O Tri-Sil Reagents –C–NH2 MTBSTFA –C–NH2 Primary MTBSTFA+TBDMCS O Acylation MBTFA O TFAA –C–NHR –C–NHR PFAA TMS Amides Difficult to form due to steric hindrance TMS Amides TMS Amides TMCS used as a catalyst TMS Amides Reaction byproducts more volatile TMS Amides TMS Amides TBDMCS Amides Difficult to form; very stable TBDMCS Amides TBDMCS aids derivatization Trifluoroacetamides Trifluoroacetamides Pentafluoropropionamides Secondary HFBA Alkylation MethElute...
Open the catalog to page 3
Derivatization Reagents for Drugs-of-Abuse Reagent Reference Amphetamines Amphetamines BSTFA 1 NH2 Amphetamines HFAA 2-5 CH Amphetamines HFAA/PFAA 6 C6H5 CH2 CH 3 Amphetamines MSTFA with TMCS 7 Amphetamines TFAA 7,8 Methamphetamine TFAA 9,10 Barbiturates BSTFA 1 MethElute Reagent (TMPAH) 11-13 O O NH Methylate Reagent (DMFDMA) 14,15 CH2 CH CH2 PFBBr 16 CH3 Cocaine Benzoylecgonine CH3 N COOH BSTFA/Butyl Iodine/TMAH 17 BSTFA 1,18 MTBSTFA 19 PFAA/PFPOH 9,20 BSA 21 BSTFA 22 MSTFA 21 TFAI 23 Marijuana THC metabolites COOH OH BSA 24 BSTFA/BSTFA+1% TMCS 24-27 BSTFA/TMCS/TMSI 24 MSTFA 9...
Open the catalog to page 4
References 1. Dutt, M.C. (1982). J. Chromatogr. 248, 115-124. 2. Wu, A.H.B., et al. (1992). J. Anal. Toxicol. 16, 137-141. 3. Thurman, E.M., et al. (1992). J. Anal. Toxicol. 16, 19-27. 4. Reimer, M.L., et al. (1993). Biol. Mass Spectrom. 22, 235-242. 5. Yamamoto, T., et al. (1989). J. Anal. Toxicol. 13, 117-119. 6. Wu, A.H.B., et al. (1992). Biol. Mass Spectrom. 21, 278-284. 7. ood, H.D. and Knitter, J.A. (1991). Capillary Chromatography. R W.G. Jennings, ed., pp. 115-130. 8. DePace, A., et al. (1990). J. Forensic Sci. 35(6), 1431-1435. 9. Mulé, S.J. and Casella, G.A....
Open the catalog to page 5
Introduction to Gas Chromatography Reagents Efficiency Direct analysis can be difficult when compounds interact with each other or with the column. These interactions can lead to poor peak resolution and/or asymmetrical peaks that make proper peak integration difficult or impractical. This interference can be reduced with conversion to derivatized products.13,14 Compounds that exhibit co-elution can often be separated by using the appropriate derivatization methods. Detectability As demand increases for the analysis of increasingly smaller amounts of materials, it becomes important to...
Open the catalog to page 6
Thermo Scientific Silylation Reagents Silylation and Silylation Reagents Only Thermo Scientific Reagents offer the combination of variety, quality and reliability. Silyl derivatives are the most widely used derivatives for gas chromatographic applications. Usually they are formed by the replacement of the active hydrogens from acids, alcohols, thiols, amines, amides and enolizable ketones and aldehydes with the trimethylsilyl group. A variety of reagents is available for the introduction of the trimethylsilyl group. These reagents differ in their reactivity, selectivity and side reactions...
Open the catalog to page 7
Thermo Scientific Silylation Reagents For excellent chromatographic separations. The reagent to choose for difficult-to-silylate compounds. CH 3 BSTFA is a powerful trimethylsilyl donor, with donor strength that is comparable to its unfluorinated analog BSA [N,O-Bis(trimethylsilyl) acetamide]. BSTFA reacts to replace labile hydrogens on a wide range of polar compounds with a -Si(CH3)3 group. This physical characteristic is particularly useful in the gas chromatography of some lower boiling TMS-amino acids and TMS Krebs cycle acids. PROTOCOL Thermo Scientific BSTFA + 1% TMCS is ideal for...
Open the catalog to page 8
MSTFA and MSTFA 1% TMCS Offers maximum volatility. The perfect reagent for volatile TMS derivatives. CH 3 Highlights: • Trimethylsilyl donor strength comparable to BSA and BSTFA • Reacts to replace labile hydrogens on a wide range of polar compounds with a -Si(CH3)3 group • Used to prepare volatile and thermally stable derivatives for GC and MS • Primary advantage of Thermo Scientific MSTFA is the volatility of its byproduct, N-methyltrifluoroacetamide; MSTFA is the most volatile TMS-amide available which has an even lower retention time than MSTFA • Often TMS derivatives of small molecules...
Open the catalog to page 9
Thermo Scientific Silylation Reagents MTBSTFA and MTBSTFA + 1% TBDMCS Offers stable TBDMS (tert-butyldimethylsilyl) derivatization. The strongest hydroxyl silylator available for carbohydrates and steroids. Sakauchi and Horning have shown TMSI to be an all-purpose reagent for unhindered steroids to highly hindered steroids. Highlights: • Derivatizes hydroxyl, carboxyl, thiol and primary and secondary amines • Typical yields are >96% • Provides TBDMS ethers that are 104 times more stable to hydrolysis than TMS ethers • Reaction byproducts are neutral and volatile • Derivatives have a high...
Open the catalog to page 10
The popular choice for silylation of sugars and related substances. An excellent catalyst for difficult-to-silylate compounds. Thermo Scientific HMDS greatly extends the practical range of GC, improving chromatographic results in the silylation of sugars A critical study of the optimal proportions of HMDS and trimethyl- chlorosilane for producing maximum yield of trimethylsilyl derivatives was conducted by Sweeley, etal. This protocol describes the method of Sweeley, etal. for the trimethylsilylation of sugars and related substances. 1. Combine 10 mg or less carbohydrate sample, 1.0 ml...
Open the catalog to page 11All Thermo Scientific catalogs and technical brochures
-
CryoStar NX50
2 Pages
-
MSC-Advantage BSC
54 Pages
-
Multifuge
58 Pages
-
Thermo Scientific Pico 17 / 21
52 Pages
-
CO2 Incubator HERAcell 150 i / 240 i
196 Pages
-
Revco® Blood Bank Refrigerators
37 Pages
-
Multidrop Combi nL
4 Pages
-
Forma Environmental Chambers
25 Pages
-
MaxQ 6000
40 Pages
-
Multi Tube Rotator
13 Pages
-
picoSpin Spectrometer
6 Pages
-
1300 Series A2
76 Pages
-
CO2 Incubator Family
28 Pages
-
CO2 incubators
7 Pages
-
MaxQ Mini 4450 Shaker
40 Pages
-
E1-ClipTip Electronic Pipette
108 Pages
-
Varioskan™ LUX
54 Pages
-
ISQ Series Mass Spectrometers
412 Pages
-
Q Exactive™ BioPharma
16 Pages
-
Q Exactive Plus
4 Pages
-
LTQ XL™
4 Pages
-
Platinum DNA polymerases
6 Pages
-
-80°C Benchtop Freezer
2 Pages
-
Nicolet iS10 FT-IR Spectrometer
12 Pages
-
Ion S5 and Ion S5 XL Systems
2 Pages
-
SureTect Range
4 Pages
-
QuantStudio™ 3
8 Pages
-
Furnaces
2 Pages
-
Nunc Serological Pipettes
3 Pages
-
Nunc Cell Factory systems
8 Pages
-
Vanquish Duo UHPLC System [ZH]
12 Pages
-
native MS
16 Pages
-
Compound Discoverer Software
12 Pages
-
Vanquish Horizon UHPLC System
16 Pages
-
Thermo Scientific TSQ Duo
9 Pages
-
Thermo Scientific TSQ 8000 Evo
16 Pages
-
Heraeus Labofuge 300
2 Pages
-
Heraeus Cryofuge 5500i
8 Pages
-
Heraeus Clinifuge Centrifuge
2 Pages
-
Vials and Closures Catalog 2014-2015
128 Pages
-
RM 200 EG
4 Pages
-
Darwin LIMS
2 Pages
-
Immunohistory Solutions
292 Pages
-
Process Water Products Catalog
68 Pages
-
Technical Training Catalog
15 Pages
-
FBS Alternatives
8 Pages
-
Pierce Magnetic Beads
16 Pages
-
interactive catalog today
222 Pages
-
HERAGUARD HPH clean benches
2 Pages
-
MBC 240 Bench Top Analyzer
4 Pages
-
Konelab 20
2 Pages
-
Indiko
6 Pages



























































































![ISQ 7000 Single Quadrupole GC-MS System VPI [ZH]](https://img.medicalexpo.com/pdf/repository_me/78678/isq-7000-single-quadrupole-gc-ms-system-vpi-zh-191148_1mg.jpg)
![ISQ 7000 Single Quadrupole GC-MS System [ZH]](https://img.medicalexpo.com/pdf/repository_me/78678/isq-7000-single-quadrupole-gc-ms-system-zh-191147_1mg.jpg)
![Vanquish Duo UHPLC System [ZH]](https://img.medicalexpo.com/pdf/repository_me/78678/vanquish-duo-uhplc-system-zh-191145_1mg.jpg)





















































![Thermo Scientific Herasafe KS and KSP Class II Biological Safety Cabinets [EN]](https://img.medicalexpo.com/pdf/repository_me/78678/thermo-scientific-herasafe-ks-ksp-class-ii-biological-safety-cabinets-en-85081_1mg.jpg)











