 Website:
TSI
Website:
TSI
Group: TSI
Catalog excerpts

FMS 5 FACILITY MONITORING SOFTWARE FOR LIFE SCIENCE APPLICATIONS UNDERSTANDING, ACCELERATED
Open the catalog to page 1
E AND E OF MIND REGULATORY COMPLIANCE FMS 5 is compliant environmental monitoring system software for the Pharmaceutical, Medical Device and Life Science industries. All software development activities at TSI follow the ISPE GAMP® lifecycle model. FMS 5 is assigned current GAMP® category 4 as configurable software. It is specifically intended for use where compliance to EU GMP Annex 1 and FDA cGMP aseptic processing guidance is required. FMS 5 enables compliance to the FDA 21 CFR Part 11 ruling. Full audit trail, password aging and lockout after failed logins, ensures secure, tamper proof...
Open the catalog to page 2
Ease of Use An intuitive, user configurable interface means immediate visibility of real-time data. Single mouse click access to historical data and report generation leads to reduced operator training, immediate data access and improved process control. Multi-level maps display the last recorded value for I each instrument and show real-time icons in Red for Alarm, Yellow for Warning, Green for Okay and Blue for Instrument Failure. Simply click on the instrument icon for instant, detailed information. Communication FMS 5 supports multiple system outputs like beacons, sounders, SMS text,...
Open the catalog to page 3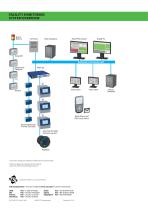
FACILITY MONITORING SYSTEM OVERVIEW Alarm Beacon Main Database Temp/RH Dedicated or Corporate LAN Differential Pressure View Client View Client Email Alerts and SMS (text) Alerts AeroTrak Remote Particle Counters AeroTrak Portable Particle Counters TSI, and the TSI logo are registered trademarks of TSI Incorporated. GAMP is a registered trademark of International Society for Pharmaceutical Engineering, Inc. TSI Incorporated - Visit our website www.tsi.com for more information. USA UK France Germany India China Singapore Mirror Database
Open the catalog to page 4All TSI catalogs and technical brochures
-
BIOTRAK®
2 Pages
-
Pharma-Life Science brochure
3 Pages
-
QCM-MOUDI Impactor Model 140
8 Pages
-
Particle Instruments Catalog US
44 Pages
-
SidePak AM520
92 Pages
-
TSI PARTICICLE TECHNOLOGY
44 Pages
-
Flexible lab control options
2 Pages
Archived catalogs
-
Mass Flow Meters for Gases
4 Pages



































