
DeNovo® NT Natural Tissue Graft Arthroscopically-Assisted Surgical Technique for Ankle Cartilage Repair
1 /
8Pages
Catalog excerpts
DeNovo NT Natural Tissue Graft ® Arthroscopically-Assisted Surgical Technique for Ankle Cartilage Repair
Open the catalog to page 1
Arthroscopically-Assisted Surgical Technique for Ankle Cartilage Repair Pre-operative Planning DeNovo NT Natural Tissue Graft is an off-the-shelf human tissue allograft, consisting of juvenile hyaline cartilage pieces with viable chondrocytes, and is intended for the repair of articular cartilage lesions in a single-stage procedure. The DeNovo NT Graft surgical technique eliminates the need for harvesting and suturing of a periosteal flap, as it employs fibrin sealant to secure the particulated tissue pieces into the lesion. Pre-operative planning may include an MRI or CT scan to better...
Open the catalog to page 2
Arthroscopically-Assisted Surgical Technique for Ankle Cartilage Repair Arthroscopy Perform a diagnostic arthroscopy to visualize the ankle lesion using a 2.7mm or 1.9mm arthroscope to inspect the lesion (Fig. 1). Removal of the distal tibial cortical bone anterior to the cartilage surface of the tibia (tibial plafondoplasty) may be performed as needed to obtain adequate visualization of posterior lesions. Fig. 1 External view of the arthroscope and internal arthroscopic view of lesion. Prepare the Cartilage Lesion Curette the lesion to a stable border (margin), removing the affected...
Open the catalog to page 3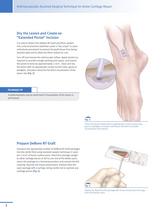
Arthroscopically-Assisted Surgical Technique for Ankle Cartilage Repair Dry the Lesion and Create an “Extended Portal” Incision It is vital to deliver the DeNovo NT Graft and fibrin sealant into a dry environment (whether under a “dry scope” or open arthrotomy procedure) to prevent the graft tissue from being washed away and to allow the fibrin sealant to cure. Turn off and remove the arthroscopic inflow. Apply traction as required to provide enough working joint space, and extend the portal incision by approximately 1-2cm . Clean and dry the lesion with an appropriate curved suction tube,...
Open the catalog to page 4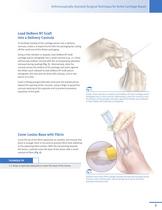
Arthroscopically-Assisted Surgical Technique for Ankle Cartilage Repair Load DeNovo NT Graft into a Delivery Cannula To facilitate loading of the cartilage pieces into a delivery cannula, create a scooped funnel with the packaging by cutting off the small end of the blister packaging. Using a Freer elevator or spatula, load DeNovo NT Graft cartilage pieces retrograde into a small cannula (e.g., a 1.9mm arthroscopy outflow cannula with the accompanying obturator removed during loading) (Fig. 5). Alternatively, slide the cannula across the bottom of the package and press against the blister...
Open the catalog to page 5
Arthroscopically-Assisted Surgical Technique for Ankle Cartilage Repair Deliver DeNovo NT Graft After about 1-2 minutes and before the fibrin fully cures, introduce the loaded cannula, and use the obturator to extrude the DeNovo NT Graft cartilage pieces into the lesion and on top of the fibrin (Fig. 7). Ensure that at least 50% of the lesion area is covered with uniformly distributed tissue pieces. In areas that are not fully shouldered, it is recommended that a gap of approximately 1mm be left between the tissue pieces and the edge of the lesion to minimize the risk of graft delamination....
Open the catalog to page 6
Arthroscopically-Assisted Surgical Technique for Ankle Cartilage Repair Final Inspection and Close Check for any extraneous debris or dislodged cartilage pieces and remove with forceps or gentle suction. Perform a standard closure with suture, and apply a splint with the ankle in a neutral position (Fig. 10). Post-operative Care General post-operative guidelines include: Final tissue/ fibrin construct • Non-weight bearing for 6 weeks – Weeks 0 -2: Posterior plaster splint with the ankle in neutral position is used until sutures are removed – Weeks 2-6: A generic removable boot brace is...
Open the catalog to page 7
This documentation is intended exclusively for physicians and is not intended for laypersons. Information on the products and procedures contained in this document is of a general nature and does not represent and does not constitute medical advice or recommendations. Because this information does not purport to constitute any diagnostic or therapeutic statement with regard to any individual medical case, each patient must be examined and advised individually, and this document does not replace the need for such examination and/or advice in whole or in part. Please refer to the package...
Open the catalog to page 8All Zimmer Biomet catalogs and technical brochures
-
MODULAR FEMORAL Revision System
14 Pages
-
A.L.P.S.®
44 Pages
-
Constrained Posterior Stabilized
12 Pages
-
Persona PERSONALIZED KNEE
7 Pages
-
Avenir® Femoral Hip System
12 Pages
-
The CLS® Spotorno® Stem
16 Pages
-
Alloclassic®Zweymüller®Stem
12 Pages
-
®Zimmer® Segmental System
6 Pages
-
NexGen® RH Knee
8 Pages
-
Persona-Partial
12 Pages
-
Zimmer Natural Nail System
8 Pages
-
tourniquet-systems-brochure
8 Pages
-
modern-cementing-technique
16 Pages
-
CoAxial Spray Kit
8 Pages
-
Biologics
24 Pages
-
PowerPump DP System
2 Pages
-
Sidus
40 Pages
-
Ankle Fix System 4.0
8 Pages
-
Anatomical Shoulder System
6 Pages
-
Fitmore Hip Stems
6 Pages
-
NexGen High-Flex Implant
8 Pages
-
Trabecular Metal ™Glenoid
4 Pages
-
Anatomical Shoulder ™ System
6 Pages
-
Zimmer ® PSI Shoulder
6 Pages
-
Zimmer personna
12 Pages
-
ZImmer iASSIST
44 Pages
-
Fitmore ® Hip Stem
24 Pages
-
Persona Knee
6 Pages
-
Trauma Solutions
10 Pages
-
Colagen Repair Patch
2 Pages
Archived catalogs
-
Fitmore® Hip Stem
6 Pages
-
Zimmer® Segmental System
6 Pages
























































































