 Website:
Aesculap®
Website:
Aesculap®
Grupo: B. Braun Melsungen
Excertos do catálogo

Aesculap® CeSpace® Titanium / PEEK Anterior Cervical Interbody Fusion System Aesculap Spine
Abrir o catálogo na página 1
E Ordering Information E1 Implants E2 Instruments
Abrir o catálogo na página 2
Foreword CeSpace is a spacer used for cervical interbody fusion. It is indicated for the treatment of degenerative diseases of the cervical disc and instabilities in the C3 to C7 region. The design of the CeSpace implant allows a maximum contact area between implant and vertebral endplates. CeSpace implants are available in PEEK and Titanium, depending on the preference of the surgeon. Stabilization with CeSpace Titanium. CeSpace stands for Primary stability Restoration of the natural disc height and lordosis and Long-term maintenance of the spinal balance. Combined with reliable...
Abrir o catálogo na página 3
CeSpace – Titanium The heart of this implant is a solid titanium alloy core (Ti6Al4V / ISO5832-3). The core is mantled with the proven Plasmapore coating to increase the area of contact between implant and endplate. Plasmapore is a pure titanium coating (Ti / ISO5832-2) which offers an optimal foundation for the ingrowth of bone due to its balanced relationship between pore depth, porosity and roughness. Using a special manufacturing procedure, the implant surface is sprayed with pure titanium powder. Molten titanium particles settle on the core of the implant where they cool rapidly,...
Abrir o catálogo na página 4
CeSpace – PEEK The material used is biocompatible PEEK-OPTIMA, which was introduced by Invibio in 1999. PEEK stands for PolyEtherEtherKetone. PEEK-OPTIMA polymer complies with ISO 10993-1, USP Class VI and ASTM F2026 for use as a medical implant material. The use of PEEK-OPTIMA as an orthopedic device material enjoys increased popularity in recent years due to the material’s unique combination of characteristics. It’s properties include radiolucency, high mechanical strength, biocompatibility and compatibility with standard sterilization methods. Of particular interest is the modulus of...
Abrir o catálogo na página 5
CeSpace® Titanium Implant Features – CeSpace Titanium Plasmapore coating: rapid and safe osteointegration High primary stability due to a rough surface High secondary stability due to a fast migration of bone cells into the Plasmapore structure Intelligent implant design Fixation crown for an exact implant fit and high primary stability Optimized ratio between contact area and opening Option of filling with bone or bone substitute to enhance bone bridging Implant variety Adequate range of sizes providing the right implant to fit the patient Minimum 4 mm height Thought-out instruments Simple...
Abrir o catálogo na página 6
CeSpace® PEEK Implant Features – CeSpace PEEK Position verification despite X-ray transparency PEEK-OPTIMA allows quick and simple assessment of the bone structure and progress towards bone fusion Rod style marker for easy and exact implant positioning and localization Intelligent implant design Anatomical shape and serrated profile for an exact implant fit and high primary stability Optimized ratio between contact area and opening Option of filling with bone or bone substitute to enhance bone bridging Implant variety Adequate range of sizes providing the right implant to fit the patient...
Abrir o catálogo na página 7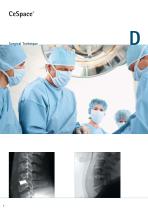
Surgical Technique
Abrir o catálogo na página 8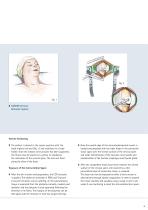
Fig. 1 ❚ CASPAR Cervical Retractor System Patient Positioning ❚ The patient is placed in the supine position with the head slightly reclined (Fig. 1) and stabilized in a head holder. Once the lordotic cervical spine has been supported, the thorax may be placed on a pillow to emphasize the reclination of the cervical spine. The arms are fixed along the sides of the body. Exposure of the Intervertebral Space ❚ After the skin incision and preparation, the CCR retractor is applied. The blades are available in PEEK and Titanium. A counter retractor can be used (Fig. 2). The subcutaneous tissue...
Abrir o catálogo na página 9
Surgical Technique ❚ CASPAR Vertebral Body Distractor ❚ CASPAR Distraction Screws Fig. 4 ❚ Trial Implants CeSpace Titanium FJ164R-FJ167R/ FJ174R-FJ177R ❚ Trial Implants CeSpace PEEK: FJ474R-FJ478R/ FJ484R-FJ488R Distraction / Discectomy / Preparation of the Endplates Implant Selection ❚ The distraction screws are placed in position and the CASPAR distractor is applied following the CASPAR technique (Fig. 3). ❚ The correct implant size can be established using the trial implants (Fig. 5). ❚ Complete discectomy is performed using various rongeurs, rectangular curettes and bone curettes (Fig....
Abrir o catálogo na página 10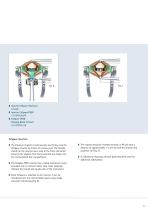
❚ Inserter CeSpace Titanium FJ100R ❚ Inserter CeSpace PEEK FJ415R/FJ497R ❚ CeSpace PEEK Packing Block / Punch FJ413P/FF914R CeSpace Insertion ❚ The Titanium implant is held securely and firmly onto the CeSpace inserter by means of a screw joint. The flexible sheath on the inserter has a stop at the front end which prevents the implant from being inserted too deeply into the intervertebral disc compartment. ❚ The implant should be inserted centrally in AP and with a distance of approximately 1-2 mm to both the anterior and posterior rim (Fig. 7). ❚ If indicated an Aesculap cervical plate...
Abrir o catálogo na página 11
CeSpace® Titanium Ordering Information – Implants Diameter CeSpace Titanium CeSpace Titanium CeSpace Titanium CeSpace Titanium CeSpace Titanium CeSpace Titanium CeSpace Titanium CeSpace Titanium Implant materials ISOTAN F Plasmapore Titanium forged alloy (Ti6Al4V / ISO 5832-3) Pure titanium (Ti / ISO 5832-2) The specified height for the lordotic implant refers to the average height, which means the anterior section of the implant is higher than the posterior section. All CeSpace implants are individually sterile pa
Abrir o catálogo na página 12
CeSpace® PEEK Ordering Information – Implants Diameter CeSpace PEEK CeSpace PEEK CeSpace PEEK CeSpace PEEK CeSpace PEEK CeSpace PEEK CeSpace PEEK CeSpace PEEK CeSpace PEEK CeSpace PEEK Implant material PEEK-OPTIMA (PolyEtherEtherKeton) Registered trademark of Invibio Ltd, Lancashire FY5 4QD, UK The specified height for the lordotic implant refers to the average height, which means the anterior section of the implant is higher than the posterior section. All CeSpace implants are individually sterile
Abrir o catálogo na página 13Todos os catálogos e folhetos técnicos Aesculap®
-
proGAV ® 2.0
36 Páginas
-
Targon® FN
36 Páginas
-
AESCULAP® NEUROENDOSCOPY
128 Páginas
-
AESCULAP® Metha®
28 Páginas
-
AESCULAP® BULLDOG CLIPS
8 Páginas
-
Quintex®
8 Páginas
-
MONOMAX®
16 Páginas
-
Premilene® Mesh Plug
8 Páginas
-
AESCULAP® Excia® 12/14
18 Páginas
-
Aesculap®
16 Páginas
-
VascuFlex®
12 Páginas
-
Aesculap Aeos®
16 Páginas
-
LC – LINEAR CUTTER STAPLER
6 Páginas
-
M.blue®
44 Páginas
-
AESCULAP® PREMIUM SOLUTION FOR VATS
48 Páginas
-
Nelson® deluxe
8 Páginas
-
CARDIOLOGY
44 Páginas
-
Aesculap® Targon® RF
28 Páginas
-
Aesculap® OrthoPilot®
52 Páginas
-
TrendHip®
16 Páginas
-
AESCULAP® Neuro-Patch® & Lyoplant®
2 Páginas
-
proSA®
30 Páginas
-
activ L®
44 Páginas
-
AESCULAP® SINGLE USE PRODUCTS
88 Páginas
-
AESCULAP® CoreHip® SYSTEM
36 Páginas
-
AdTec® bipolar
8 Páginas
-
Aesculap® Heart XS Retraction System
6 Páginas
-
Aesculap Surgical Instruments
64 Páginas
-
Aesculap® TITANIUM Micro Instruments
16 Páginas
-
EV3.0 CAMERA CONTROL UNIT
12 Páginas
-
Aesculap® EinsteinVision® 2.0
8 Páginas




































