
Excertos do catálogo

Hammertoe Implant SURGICAL TECHNIQUE
Abrir o catálogo na página 1
surgical technique presented by LOWELL SCOTT WEIL, DPM
Abrir o catálogo na página 2
HAMMERTOE IMPLANT S WANS O N hammertoe IMPLANT general PRECAUTIONS as described by Lowell Scott Weil, DPM The potential for complications or adverse reactions with any implant can be minimized by following the instructions for use provided in product literature. It is the responsibility of each surgeon using implants to consider the clinical and medical status of each patient and to be knowledgeable about all aspects of implant procedure and the potential complications that may occur. The benefits derived from implant surgery may not meet the patient’s expectations or may deteriorate with...
Abrir o catálogo na página 3
HAMMERTOE IMPLANT Some preventive measures to consider to minimize the potential for complications: • Follow guidelines for indications and contraindications provided below: • Identify prior pathology • Stabilize collapse deformities • Bone graft pre-existing cysts • Use a properly sized implant • Avoid K-wires and sutures through the implant If complications develop, possible corrective procedures include: Implant removal • Implant removal • Synovectomy • Bone grafting of cysts • Limited intertarsal fusion • Replacement of the implant • Removal of the implant with fusion of the joint...
Abrir o catálogo na página 4
HAMMERTOE IMPLANT Some degree of particle formation is inevitable with all implants including those made of silicone elastomer. The amount will vary with factors such as patient activity, metatarsal stability or instability postimplantation, implant position and the amount of soft tissue support. The patient’s biological response to these particles is variable, but can include local synovitis and bone lysis in contiguous bones. Another potential concern with silicone implants arises from case reports in the literature suggesting an association between silicone implants and immunological...
Abrir o catálogo na página 5
HAMMERTOE IMPLANT deformities, or recurrence of the original deformity. In addition, the possible complications of arthrodesis (e.g., poor positioning, incomplete fusion, etc.) as well as the postoperative fixation required for arthrodesis are minimized. A sizing set, supplied nonsterile and not suitable for implantation, is available for proper size determination during surgery. An instrument set which includes a hand reamer and a hand compactor is also available in two sizes. The reamer is used to drill the medullary canal, while the compactor is used to shape the canal to match the stem...
Abrir o catálogo na página 6
general CONTRAINDICATIONS HAMMERTOE IMPLANT • Physiologically or psychologically inadequate patient • Inadequate skin, bone, or neurovascular status • Irreparable tendon system • Possibility for conservative treatment • Growing patients with open epiphyses • Patients with high levels of activity Each patient must be evaluated by the surgeon to determine the risk/benefit relationship. surgical PROCEDURE Wright Medical Technology, Inc. does not recommend a particular surgical technique when using the implant. Proper surgical procedures and techniques are necessarily the responsibility of the...
Abrir o catálogo na página 7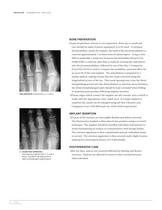
HAMMERTOE IMPLANT BONE PREPARATION Proper preparation of bone is very important. Bone size is small and care should be taken if power equipment is to be used. To prepare intramedullary canals for implant, the head of the proximal phalanx is resected approximately 7 to 8mm from its distal aspect. Using a wire, drill or preferably a small size Swanson Intramedullary Broach (Cat. #6480-0100) a centered, pilot hole is made by rotating the instrument into the proximal phalanx, followed by use of the Size 1 Compactor (Cat.#5431-0110) is used to compact the medullary canal and allow for an exact...
Abrir o catálogo na página 8
HAMMERTOE IMPLANT A bibliography may be obtained by writing Wright Medical Technology, Inc. or by contacting your Wright Medical Technology, Inc. representative. HOW SUPPLIED The Hammertoe Implant (Swanson Type) Weil Design has been sterilized and packaged as follows: TYPICAL DIMENSIONS SIZE SMALL STEM QUANTITY 1 Each, sizes: 1S, 1, 1L, 1W, 2S, 2, 2L Numerically marked, color coded (non-sterile) NOT FOR IMPLANTATION.
Abrir o catálogo na página 9
HAMMERTOE IMPLANT HANDLING AND STERILIZATION This product has been sterilized and should be considered sterile unless the package has been opened or damaged. Remove from package, using accepted sterile technique, only after the correct size has been determined. Always handle the product with powder-free gloves, and avoid contact with hard objects that may damage the product. Handling of the implant should be done with blunt instruments to avoid surface trauma or contamination with foreign bodies. Rinse the implant thoroughly with sterile saline solution before insertion. The sizing set is...
Abrir o catálogo na página 10
c Wright Medical Technology, Inc . Wright Medical Europe SA 5677 Airline Road Arlington, TN 38002 901.867.9971 phone 800.238.7188 toll-free www.wmt.com Commerce Parc Gebouw B, 6th Floor Krijgsman II 1186 DM Amsterdam The Netherlands Tel +31 (0) 205450100 ©2006, Wright Medical Technology, Inc. All Rights Reserved.
Abrir o catálogo na página 12Todos os catálogos e folhetos técnicos Wright Medical Technology
-
Amnion Case Study Series Compendium
32 Páginas
-
Focused on Biologics
2 Páginas
-
Wright Code of Business Conduct
36 Páginas
-
FORCE FIBER
4 Páginas
-
DARCO Headed Compression Screw
2 Páginas
-
BIOFOAM®
1 Páginas
-
TENSIX
2 Páginas
-
ALLOPURE
6 Páginas
-
MEDIALMAX
2 Páginas
-
DARCO™
1 Páginas
-
INVISION™
2 Páginas
-
PROPHECY
6 Páginas
-
BIOFOAM Wedge System SURGICAL TECHNIQUE
12 Páginas
-
SALVATION® External Fixation System
2 Páginas
-
SALVATION® 3Di Plating System
2 Páginas
-
SWANSON Flexible Hinge Toe
1 Páginas
-
Comprehensive Osteogenic Solutions
4 Páginas
-
css
4 Páginas
-
CL AW ® II
20 Páginas
-
LMH Lesser Metatarsal Head Implant
2 Páginas
-
Hemi Phalangeal Implant
2 Páginas
-
EVOLVE PROLINE
2 Páginas
-
EVOLVE Elbow Plating System (EPS)
2 Páginas
-
ALLOMATRIX C Brochure
2 Páginas
-
UDIN Surgical Technique ? SO328?907
4 Páginas
-
PRO?TOE? VO Sales Sheet ? FA634?1110
1 Páginas
-
DARTFIRE® Small Screw System ? FA332?609
16 Páginas
-
Darco MFS ? SO117?407
8 Páginas
-
Darco MRS ? SO118?407
8 Páginas
-
Darco LPS sales sheet ? FA474?1207
1 Páginas














































